Supporting Ontario Hospitals in Health Canada Inspections: An ORBCoN Initiative
Written by: Kimberly Schonewille- ORBCoN NE Project Coordinator
In 2018, Health Canada (HC) broadened its inspection scope to include both registered and non-registered establishments, emphasizing the need for compliance within the Blood Regulations across all Canadian healthcare facilities that handle blood components. In response, the Ontario Regional Blood Coordinating Network (ORBCoN) launched a strategic initiative to support Ontario Transfusion Medicine Laboratories (TML), with compliance to the Blood Regulations, in preparing for HC inspections, and an overall goal in ensuring safer transfusion practices province wide.
Background
In October 2014, Health Canada fulfilled their final obligations to the recommendations put forward from the Krever Commission, which investigated Canada’s tainted blood scandal of the 1980’s. This final recommendation was to legislate regulations that apply to human blood collected for transfusion or further manufacture into drugs for human use. The Blood Regulations introduce specific safety requirements for whole blood and its components while consolidating existing blood safety rules in other parts of the Food and Drug Regulations. These regulations apply to all establishments who are involved in any capacity in the processing, labeling, storing, distributing (or redistributing), transforming or importing blood components. The regulations fall under the Food and Drug Act which allows for enforcement and inspections which are performed by Health Canada auditors. Establishments (i.e., Transfusion Medicine Laboratories) were to review their processes and determine their need to register with Health Canada or remain as a non-registered establishment.1
Identifying the Need for Support
With the expansion of HC inspections to a wider range of establishments, a gap in awareness among TML leaders, particularly among non-registered sites was implied. A 2022 provincial stakeholder meeting identified key areas requiring support and proposed the development of targeted resources. ORBCoN, acting as a liaison between HC, the Ministry of Health (MOH), and Ontario hospitals, took the lead in coordinating efforts to address these needs amongst these groups.
Strategic Use of HC Inspection Reports
ORBCoN leverages HC inspection observation reports to drive improvements in five key areas:
1. Risk Identification and Mitigation
- Analyzing inspection reports to pinpoint common blood component safety risks.
- Developing and publishing education initiatives to proactively address these risks and enhance patient safety.
2. Error Tracking and Reporting
- Promoting standardized processes for reporting transfusion-related errors and injuries.
- Improving consistency and awareness across Ontario’s healthcare system.
3. Regulatory Monitoring and Support
- Keeping stakeholders informed of HC tools and updates.
- Tracking Exit Notices and Final Inspection Reports to identify provincial trends.
4. Education and Resources
- Developing and distributing resources such as internal audit tools (e.g., checklists for auditors, audit plans and example schedules, as well as audit report templates), annual report templates, Blood regulations competency assessments, and a one-page summary for non-registered sites.
- Summarizing inspection findings and recognizing trends to guide hospital preparation and further develop resources.
- Creating and presenting a Health Canada specific slide during annual hospital site visits to send a consistent message provincial-wide.
5. Stakeholder Engagement
- Facilitating the Provincial Blood Regulation Peer Working Group (BRWG) to collaboratively review inspection reports, create or revise compliance tools.
Our Goal
The aim is to improve compliance to Health Canada Blood Regulations, standardize practices across Ontario hospitals and enhance patient safety in transfusion medicine.
This initiative exemplifies ORBCoN’s commitment to fostering a culture of safety, collaboration, and continuous improvement in transfusion medicine across Ontario. By proactively addressing regulatory challenges and supporting hospitals through education and engagement, ORBCoN is helping to ensure that all sites, registered or non-registered, are prepared for Health Canada inspections and aligned with national standards.
ORBCoN’s Annual Report of Health Canada Observations
Fig.1- Observations by Health Canada in Ontario Hospitals 2024-25
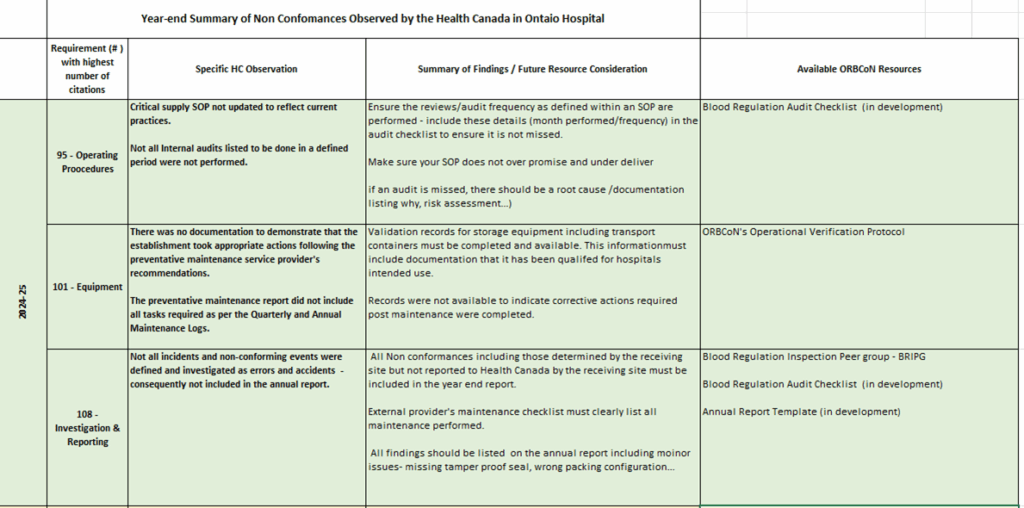
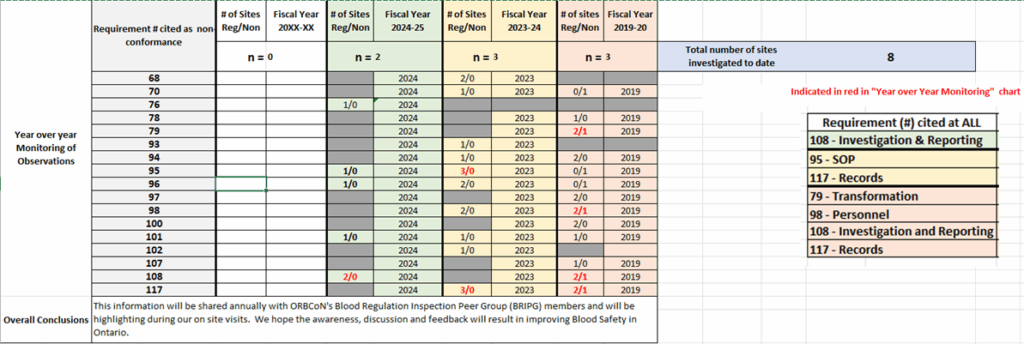
Fig.2 – Overview of Observations by Health Canada in Ontario Hospitals 2019-2025
Reference:
Health Canada. Guidance document: Blood regulations [Internet]. Ottawa: Government of Canada; [cited 2025 Sep 4]. Available from: https://www.canada.ca/en/health-canada/services/drugs-health-products/biologics-radiopharmaceuticals-genetic-therapies/applications-submissions/guidance-documents/blood-regulations/guidance-document-blood-regulations.html#a1.1