Transfusion Medicine Boot Camp for Nurses
Donna Berta RN BScN, Clinical Project Coordinator – Nursing, ORBCoN
Background
ORBCoN’s annual educational event, Transfusion Medicine Boot Camp for Nurses, originated in 2018. The intent is to foster transfusion knowledge and skills for nurses (broadened to transfusionists, health care professionals administering blood) to ensure blood is administered safely to patients. A volunteer working group comprised of a transfusion medicine physician, a transfusion safety nurse, clinical nurse educators, a patient blood management coordinator, and ORBCoN staff organize the event. Year over year attendee feedback has guided the event’s format and program content.
A consistently requested feature has been interactive learning. Speakers are obliged to employ pre/post transfusion knowledge questions (polling the attendees prior to the presentation, providing the answer information during their presentation, and repeat polling the attendees following the presentation with review of the correct answers). As well, speakers are encouraged to provide patient case-based sessions with additional polling questions to support critical thinking and problem solving.
2022 Program
On November 23, 2022, a no cost to attendees, virtual, mandatory registration (for security purposes) three-hour educational session was presented. The program incorporated plenary sessions based on Transfusion Guidelines/Blood Administration and Transfusion Reactions followed by a concurrent session with a choice of What’s Happening in Transfusion Medicine issues. The session titles included:
Plenary
- RBC Transfusions: Historical versus Best Practices
- Documenting Transfusion: Dotting the i’s, Crossing the t’s
- Transfusion Associated Circulatory Overload (TACO) Clinical Perspectives
Concurrent
- Massive Hemorrhage Protocol – Adults
- Massive Hemorrhage Protocol – Paediatrics
- All About Anemia and Iron
All sessions were recorded and posted to the ORBCoN website. Session can be viewed at https://transfusionontario.org/category/orbcon-resources/presentation-library/transfusion-medicine-boot-camp-for-nurses/.
2022 Registrants
The event included 787 registrants, primarily Ontarians. Approximately 5.6 % of registrants were from outside Ontario, including five international registrants.
The role of the registrants is summarized in Figure 1 and more specifically:
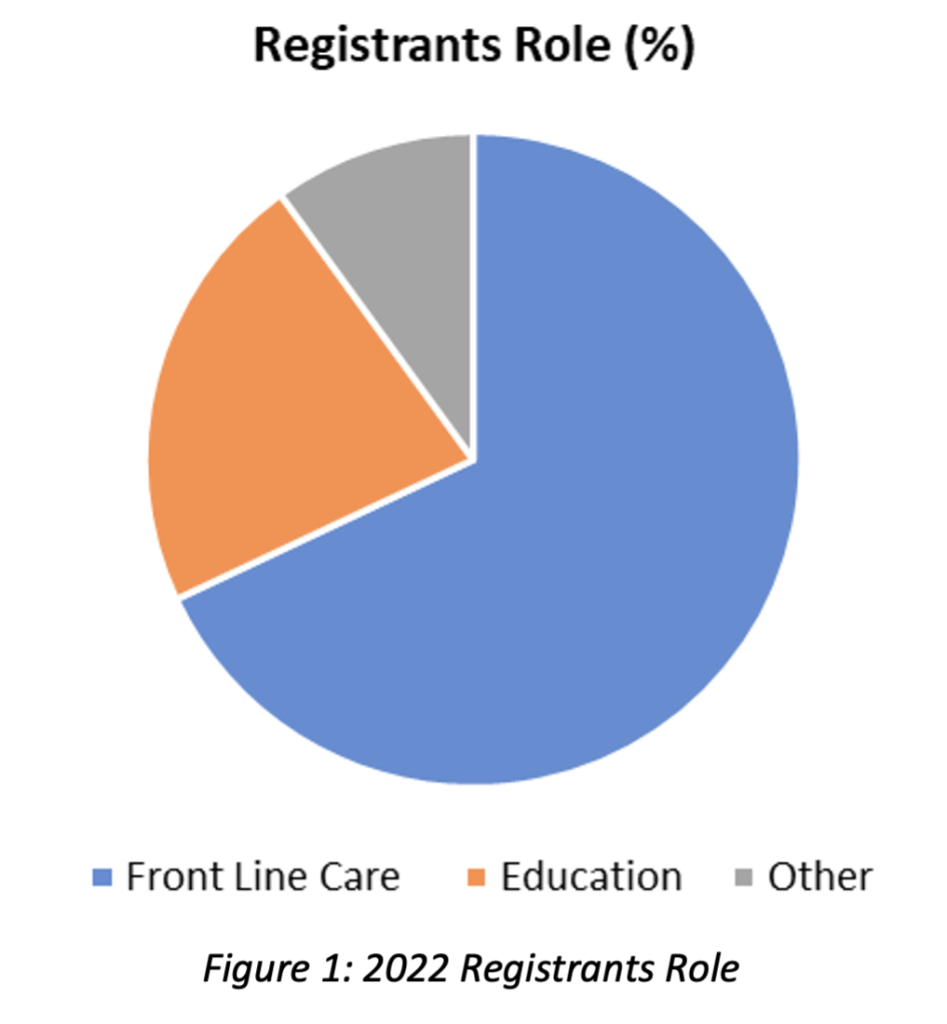
- 68 % front line nursing care provider (registered nurse, registered practical nurse, nurse practitioner, clinical nurse specialist, patient blood management coordinator)
- 22 % providing transfusion education (clinical nurse educator, nursing professional practice, transfusion safety nurse/officer)
- 10 % other (laboratory staff [technologists], manager, perfusionist, respiratory therapist, anesthesia assistant, physician, university/ college faculty, student, clinical informatics)
2022 Outcomes
Attendees provided their feedback via the evaluation survey which demonstrated:
overall appraisal: 80% excellent, 19% good after attending this event, my practice will change: 80% yes
future considerations: additional time allocated to attendee questions, more patient scenarios and polling questions, demonstrations of administration practices and equipment, provide education more than once a year potential topics of interest: IVIG, ABO Rh compatibility and antibodies, pediatrics and neonates, transfusion reactions (signs and symptoms, medications to treat)
Moving Forward
Based on previous years feedback (and reiterated in 2022), continuing education on safe blood administration is both necessary and welcomed. ORBCoN is responding by launching quarterly interactive sessions, Transfusionists Talk – Transfusion Made Bloody Easy. For details, see the announcement included in this Newsletter.
From ORBCoN Crew
TM New Year’s Resolution
A New Year is upon us and with the New Year comes time for new beginnings. New beginnings bring to mind resolutions and TM departments are always striving to improve their services which is what resolutions are all about!
ORBCoN would like to suggest some New Year’s resolutions for your TM department. These are based on the top Accreditation Canada Diagnostic non-conformances that were noticed from the previous year
This year Transfusion Medicine resolves to:
WANT: Have a training program and competency assessment process that is evaluated for its effectiveness for all personnel involved in the administration of blood products including physicians, nurses, and laboratory personnel, that will consider the complexity of tasks and potential for error.
NEED: Equipment that have the capacity to monitor the temperature range of the contents, can be set at the acceptable temperature range for the products stored inside and have an audible alarm when temperatures go beyond the acceptable range.
SHARE: Procedures for the return of products from a clinical area that take into consideration all possible products that can be issued, not only red blood cell units.
SUCCEED: Establish a comprehensive home infusion process that includes all necessary elements such as identification of person picking-up the product, redistribution of products near expiry, safety precautions, sources of variability, and follow up for transfusion complications.
Newsboard
Tech Assessment

The Bloody Easy Technologist Assessment program is now available on the new ORBCoN Learning Management System (LMS) and Surge Learning platforms.
A communication has been issued with instructions to provide site administrator information for access to the ORBCoN LMS and guidance for current Surge Learning clients.
If you have any questions or have not received the communication please reach out to alison.wendt@sunnybrook.ca
New! Save the Dates
Transfusionists Talk: Transfusion Made Bloody Easy

More info to follow! To submit a topic or patient case, email: bertad@mcmaster.ca
Featured Resources
Bloody Easy 5

Patient Pamphlet: New Version
Upcoming Events
UofT TM Rounds

January 26, 2023 @12pm-1pm
Marginalized communities in trauma research by Dr. Barbara Haas