Intravenous albumin for pediatric and adult patients
Attachment(s)
Abby Wolfe, Knowledge Broker, Canadian Blood Services
Intravenous albumin is a human-derived blood product manufactured from donated plasma. The vast majority of the plasma used to manufacture albumin for Canadian patients is collected outside of Canada. No albumin product is currently manufactured in Canada. It is administered in a wide range of clinical settings with highly variable practice between regions and can be associated with adverse consequences including fluid overload, hypotension, hemodilution requiring red blood cell transfusion and anaphylaxis. Intravenous albumin is also more expensive to manufacture compared to alternatives such as crystalloids, and very few indications have an evidence base that supports its routine use in practice to improve patient outcomes.
Towards the vision of “The right transfusion, always, everywhere”, the International Collaboration for Transfusion Medicine Guidelines (ICTMG) convened an international panel of expert volunteers including researchers, clinicians, methodologists and a patient representative to publish a new guideline on Use of Intravenous Albumin that can inform health-care professionals’ decisions on the use of albumin in its most common clinical settings. Funding to develop this guideline was provided by the Ontario Regional Blood Coordinating Network (ORBCoN) and the ICTMG, which is supported by Canadian Blood Services.
Published in the peer-reviewed journal, CHEST (2024), this guideline provides actionable and evidence-based recommendations for appropriate use of albumin in adult and pediatric patient populations undergoing cardiovascular surgery, kidney replacement therapy, complications of cirrhosis or critical illness (e.g., intensive care, sepsis). With the completion of a systematic literature review and meta-analysis, guideline recommendations were developed and reported using a rigorous methodology in accordance with Grading of Recommendations Assessment, Development and Evaluation (GRADE), Cochrane risk of bias instrument, AMSTAR, and the AGREE checklist.
Notably, twelve of the fourteen total recommendations do not support routine use of albumin. A commentary on the use of albumin in critically ill patients has also been published in CHEST Physician, including a figure outlining harmful, uncertain, and beneficial clinical settings for albumin use.
- Read the full commentary here: Use of albumin in critically ill patients | CHEST Physician (mdedge.com).
Calls for future research identified in the guideline also acknowledge a need for further evidence on dosing strategies with different albumin formulations, adverse reactions from intravenous albumin that can inform conclusions regarding potential risks, and the therapeutic targets of albumin resuscitation.
The guideline is intended primarily to inform critical care physicians, hematologists, transfusion medicine physicians, gastroenterologists, pathologists, nephrologists, hepatologists, anesthesiologists, cardiovascular surgeons, general internists, hospitalists, pharmacists and laboratory technologists, as well as medical trainees, researchers, patients and families.
Following this guideline publication, the ICTMG is undertaking a 1-year knowledge mobilization (KM) project to support the uptake of guideline recommendations into clinical practice by developing and utilizing knowledge mobilization resources, tools, and strategies. To learn more about the progress of this project, visit ICTMG.org/albumin where resources and updates will be posted as they become available. Examples of items undertaken to date include:
- A July 2024 Journal Club webcast presented in partnership with ISBT to review the guideline’s recommendations and evidence which informed the guideline’s development.
- A Breakthroughs in Blood webinar featuring panel discussion hosted by Canadian Blood Services in September 2024 with webinar recording and accompanying Mythbusters resource.
- A podcast episode recorded for the Critical Matters Podcast hosted by Dr. Sergio Zanotti.
Concurrently, ICTMG is also partnering with the Ottawa Hospital Research Institute (OHRI) on an implementation research project to understand the barriers and enablers that influence the uptake of specific guideline recommendations among Canadian health-care providers. To learn more about the guideline, available resources, and the ICTMG’s Knowledge Mobilization and Implementation Research projects, visit the ICTMG website or contact ICTMG directly via info@ictmg.org.
Featured Resource
Bedside Audit of Blood Administration Tools
Transfusionists Talk Archive
Upcoming Events
UofT Rounds

October 24, 2024 @12pm-1pm
How do we improve transfusion practice in pediatric massive hemorrhage protocols?
Dr. Kimmo Murto
Subscribe to U of T Transfusion Medicine Rounds mailing list to get registration details
Attachment(s)
Tracy Cameron, MLT, T. (Dorien) Ruijs, MD FRCPC GenPath, Donna Berta, RN, Alison Wendt MLT – Ontario Regional Blood Coordinating Network
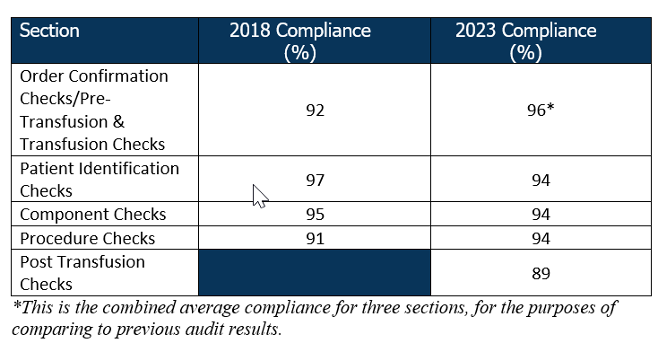
The provincial Bedside Audit of Blood Administration was conducted from January to April 2023 to assess compliance with current standards and best practices for pre-transfusion. The sections of the audit included, demographics, pre-transfusion checks (for transfusionist, transfusion service and transfusion), patient identification checks, blood component verification checks and post transfusion checks. The audit results were compared to the 2018 to measure improvement in compliance. The goal of this audit was to promote quality improvement and transfusion safety.
Notable Improvements: The 2023 audit revealed that compliance in pre-transfusion checks (96%) and procedure checks (94%) has steadily improved across the province
Areas for Growth:
Despite improvements, there are still gaps in patient identification and component verification procedure checks. Component Check compliance decreased by 1%, while Patient Identification Checks decreased by 3%. A new section was introduced in the 2023 audit that addressed Post Transfusion Checks, which showed the lowest compliance (89%) out of all sections.
Ward/Area Variations:
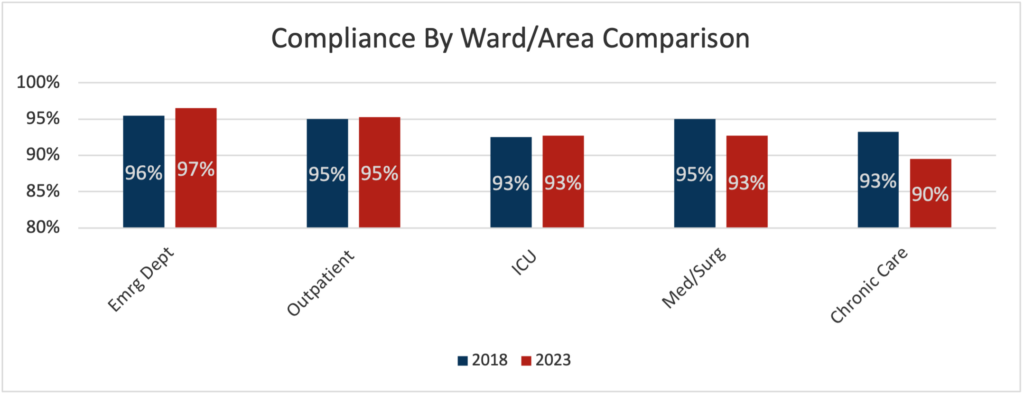
A breakdown by ward/area revealed that overall compliance levels vary significantly, with some hospital areas showing near perfect compliance while others lag. Comparison across wards/areas is limited by sample size.
Looking Ahead:
Although 100% compliance is ideal for minimizing health risks, it is rarely achieved in practice. The published report will use a “traffic light” system to assess compliance using arbitrary numerical values assigned: 100% is vibrant green (optimal), ≥95-99% is pale green (acceptable for the purposes of the report but suboptimal), 91-94% is amber (cautious observation), and ≤90% is red (alert – requires investigation). Compliance below 100%, despite a green or amber designation, may warrant further investigation and follow-up, in accordance with policies and procedures.
Data from the audit, including breakdown of compliance charts and graphs, along with a discussion on practice gaps, possible contributing factors and suggestions to address/mitigate these gaps will be made available in the full report in November 2024 on our website. Work has begun on resources to address gaps in compliance and enhance transfusion safety. These resources will be available in 2025. Look for them on our website under the Bedside Audit of Blood Transfusion (BABA) webpage.
The audit highlighted the importance for the continued need for ongoing education and process evaluation in the administration of blood components. Hospitals are encouraged to use the audit tool as a quality improvement instrument, supporting patient safety and aligning with accreditation requirements.
Ontario Hospitals: If your site is interested in performing periodic audits at the bedside and have not registered, please complete the “contact us” form on the Bedside Audit of Blood Transfusion web page.
Featured Resource
Bedside Audit of Blood Administration Tools

Transfusionists Talk Archive

Upcoming Events
UofT Rounds

October 24, 2024 @12pm-1pm
How do we improve transfusion practice in pediatric massive hemorrhage protocols?
Dr. Kimmo Murto
Subscribe to U of T Transfusion Medicine Rounds mailing list
Attachment(s)
Presented by: Dr. Anne Ellis
Evaluation Survey Link: https://orbcon.limesurvey.net/281864?lang=en
Attachment(s)
Donna Berta, RN, BScN
Access Pre & Post Transfusion Knowledge Questions and Answer with Rationale here.
Attachment(s)
https://transfusionontario.org/wp-content/uploads/2024/09/Transfusionists-Talk-2024-Sep.pdfAttachment(s)
Amanda VanSpronsen, MLT, PhD
Associate Professor, University of Alberta
Choosing Wisely Canada (CWC) aims to reduce unnecessary medical tests, procedures, and treatments. They support the development and publication of practice recommendations from numerous medical societies. In 2020, the Canadian Society for Medical Laboratory Science (CSMLS) submitted its first set of seven recommendations for CWC. As part of CWC requirements, these recommendations are focused within the scope of practice of medical laboratory professionals (MLPs), primarily Medical Laboratory Technologists and Assistants. In 2024, CWC accepted another seven recommendations from the CSMLS, bringing the total to fourteen. All recommendations published with CWC start with the word “Don’t…” but they are not hard and fast rules. They serve as cues meant to prompt reflection and support conversations about change or concerns in the workplace.
The first set of recommendations was quite general and applicable across multiple areas of practice. For example, recommendation #2 is “Don’t proceed with testing or reporting when sample quality or identification is suspect.” The new set of recommendations introduces more discipline-specific items and highlights the collaborative role MLPs play in reducing unnecessary tests and procedures. Readers may be particularly interested in recommendation #11: “Don’t process transfusion orders that do not adhere to best practices without discussing with the ordering clinician.” As all MLPs know, blood products need careful stewardship due to their finite nature and potential to cause patient harm. Many jurisdictions have enacted restrictive, evidence-based transfusion guidelines, but inappropriate orders still occur.
For instance, two studies of several Canadian hospitals showed that almost 19% of red blood cell transfusion orders [1] and just over 41% of adult platelet transfusion orders [2] were inappropriate. In both studies, researchers concluded that MLT involvement in reviewing orders added value. All stakeholders are accountable for ensuring guidelines are followed. As testing-process experts, MLPs can play a key role in screening transfusion orders and liaising with laboratory physicians, other MLPs, and ordering clinicians to make sure the test order is appropriate. With this new CWC recommendation, MLPs have even more backing when advocating for practices and programs that decrease inappropriate transfusions.
All of the CWC recommendations for Medical Laboratory Science, along with justification and supporting evidence, can be found on the Choosing Wisely Canada [3] and Lab Wisely (CSMLS) [4] websites. Understanding and applying these recommendations will help MLPs ensure responsible use of medical resources in the quest to provide the highest quality care.
References:
1. Kron AT, Collins A, Cserti-Gazdewich C, Pendergrast J, Webert K, Lieberman L, et al. A prospective multi-faceted interventional study of blood bank technologist screening of red blood cell transfusion orders: The START study. Transfusion. 2021 Feb;61(2):410-422. doi: 10.1111/trf.16243.
2. Hill-Strathy M, Pinkerton PH, Thompson TA, Wendt A, Collins A, Cohen R, et al. Evaluating the appropriateness of platelet transfusions compared with evidence-based platelet guidelines: An audit of platelet transfusions at 57 hospitals. Transfusion. 2021 Jan;61(1):57-71. doi: 10.1111/trf.16134.
3. Choosing Wisely Canada. Medical Laboratory Science. Fourteen tests and treatments to question. [Internet]. Available from: https://choosingwiselycanada.org/recommendation/medical-laboratory-science/
4. Canadian Society for Medical Laboratory Science. Lab Wisely. Recommendations Made by You. [Internet]. Available from: https://labwisely.ca/medical-laboratory-profession-cwc-recommendations/
Upcoming Events

Don’t miss our final Transfusionists Talk of the year, happening on Wednesday, September 18th where we will answer your IVIG questions!
Visit our Events page to learn more and register!
GHEST 2024


September 26, 2024 @12pm-1pm
Pre-transfusion medications for reaction prevention and treatment
Subscribe to U of T Transfusion Medicine Rounds mailing list to get registration detai
Attachment(s)
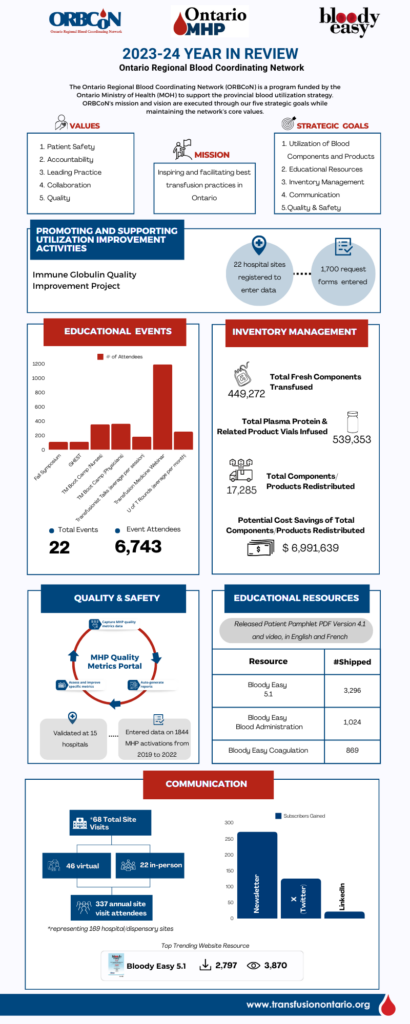
This infographic provides a snapshot of ORBCoN’s performance for the fiscal year 2023-24 and captures the most important metrics and accomplishments that reflect our company’s resilience and commitment to excellence. Dive into the numbers and insights that showcase our success over the past year.
Featured Resources
Upcoming Events

Don’t miss our final Transfusionists Talk of the year, happening on Wednesday, September 18th where we will answer your IVIG questions!
Visit our Events page to learn more and register!
GHEST 2024


September 26, 2024 @12pm-1pm
Pre-transfusion medications for reaction prevention and treatment
Subscribe to U of T Transfusion Medicine Rounds mailing list to get registration details
Attachment(s)
Donna Berta, RN, BScN
Access Pre & Post Transfusion Knowledge Questions and Answer with Rationale here
Attachment(s)
https://transfusionontario.org/wp-content/uploads/2024/06/Transfusionists-Talk-2024-Jun_Reaction-Fever.pdfDeborah Flowers, MLT, Charge Technologist Transfusion Medicine, EORLA
Most medical laboratory professionals are accustomed to the constant codes that sound overhead. On October 27, 2023, the Ottawa Hospital General Campus called an unprecedented Code Red, Code Orange and Code Grey, all within a few hours. Our team worked extremely well together under stress and solved emerging problems quickly, but could things have gone better? More importantly, what did we learn from our missteps?
Shortly before 4 pm, a Code Red was announced, and we were on emergency power. Not a cause for concern since critical areas like Transfusion Medicine are equipped with UPSs and an abundance of emergency plugs. Moments later, the lights flickered oddly, and a strange smell overtook the laboratory. In the days preceding, we had experienced several codes however this was different; it felt different. Someone suggested we prepare for evacuation, so we packed products into our Code Orange boxes and transferred the calls to the portable phone. When the notice came, we were ready. We grabbed our blood boxes, walked down the stairs, and set up in the Emergency Department garage. It wasn’t ideal but we were functional. We took inventory of the items we were missing, a charger for the portable phone, our external phone list, a pen. Small items that would have made our situation easier. Our first product request was for platelets which we hadn’t even thought to bring with us.
The Code Orange was next. Many of us have been involved in External Code Orange scenarios however never an internal disaster. It was surreal to see the Emergency Department deserted except for Emergency Response Personnel. Ambulances were diverted, patients were evacuated, transferred and discharged. We had already initiated our Code Orange policy, making it work for an internal disaster, so we continued.
Then a Code Grey was called, and we learned that emergency power had been lost to some parts of the hospital. No one could have imagined we would need a back-up for the back-up system. The next day when it was safe to return to the laboratory, it was an eerie scene. The smell was overpowering, and soot covered everything. The disaster cleanup would eventually take months to complete but we were back in Transfusion Medicine 24 hours after the first alarm sounded.
I don’t suggest laboratories plan to relocate as we did. In our situation, it was safe to bring blood products with us; we had time before the evacuation came. What was the most important lesson we learned? Effective communication is crucial in an infrastructure failure. In the beginning, our information was collected around a dying cell phone listening to situational updates. My advice to all medical laboratory professionals – review your contingency plan and recognize that communication is crucial. As Arthur Conan Doyle said, “It is easy to be wise after the event.”
Featured Resources
MHP Quality Metrics Portal
Note: Each Ontario hospital will need to determine where the data for the metrics is housed within their facility and who will be responsible for gathering and entering the data.

Upcoming Events
Transfusionists Talk

GHEST 2024 Save the Date

U of T

June 20, 2024 @12pm-1pm
MLT Session – Interesting serology cases
Subscribe to U of T Transfusion Medicine Rounds mailing list to get register