Octaplex® Human Prothrombin Complex Product Monograph
Attachment(s)
https://transfusionontario.org/wp-content/uploads/2025/11/octaplex-pm-en_Mix2Vial.pdfAttachment(s)
https://transfusionontario.org/wp-content/uploads/2025/11/fibryga_pm_en_25_nov_2021_Octajet.pdfThis electronic learning program provides health care professionals information and learning activities regarding evidence informed, best practices for FC and PCC reconstitution in clinical settings (outside of the Transfusion Medicine Laboratory). Successful completion of the accompanying assessment quiz documents competency as required by Canadian Transfusion Medicine Standards.
NOTE: If you are needing to complete the Reconstitution Outside TML Bloody Easy eLearning, please reach out to the site administrator at your facility.
If you are a site administrator and need access to the LMS, or if you do not know who your site administrator is, contact Troy Thompson at troy.thompson@sunnybrook.ca
Attachment(s)
This resource aligns with the content of the eLearning tool (evidence informed best practices for FC and PCC reconstitution). It offers quick and easy access to the eLearning information. Also, it can be downloaded and customized to your hospital’s orientation / continuing education programs.
Attachment(s)
https://transfusionontario.org/wp-content/uploads/2025/06/A-Reconstitution-FC-PCC-outside-of-TML_v8_website_final-2.pdfThese files are intended for learning management systems. For those wanting to access to the ORBCoN LMS as a site administrator, please contact troy.thompson@sunnybrook.ca.
Reconstitution Outside TML is an electronic learning tool that was developed with input from Nurses, Medical Laboratory Technologists (Transfusion Medicine), Transfusion Safety Officers across Ontario. The content reflects evidence informed best practices.
Attachment(s)
https://transfusionontario.org/wp-content/uploads/2025/06/Reconstitution-2025-NO-QUIZ-SCORM-1.2-1.zipThese files are intended for learning management systems. For those wanting to access to the ORBCoN LMS as a site administrator, please contact troy.thompson@sunnybrook.ca.
Reconstitution Outside TML is an electronic learning tool that was developed with input from Nurses, Medical Laboratory Technologists (Transfusion Medicine), Transfusion Safety Officers across Ontario. The content reflects evidence informed best practices.
Attachment(s)
https://transfusionontario.org/wp-content/uploads/2025/06/Reconstitution-2025-WITH-QUIZ-SCORM-1.2.zipThese files are intended for learning management systems. For those wanting to access to the ORBCoN LMS as a site administrator, please contact troy.thompson@sunnybrook.ca.
Reconstitution Outside TML is an electronic learning tool that was developed with input from Nurses, Medical Laboratory Technologists (Transfusion Medicine), Transfusion Safety Officers across Ontario. The content reflects evidence informed best practices.
Attachment(s)
https://transfusionontario.org/wp-content/uploads/2025/06/Reconstitution-2025-NO-QUIZ-SCORM-2004-1.zipThese files are intended for learning management systems. For those wanting to access to the ORBCoN LMS as a site administrator, please contact troy.thompson@sunnybrook.ca.
Reconstitution Outside TML is an electronic learning tool that was developed with input from Nurses, Medical Laboratory Technologists (Transfusion Medicine), Transfusion Safety Officers across Ontario. The content reflects evidence informed best practices.
Attachment(s)
https://transfusionontario.org/wp-content/uploads/2025/06/Reconstitution-2025-WITH-QUIZ-SCORM-2004.zipDonna Berta, RN, BScN
Access responses for the additional Q&A here
Pre & Post Transfusion Knowledge Questions and Answers with Rationale here
Attachment(s)
https://transfusionontario.org/wp-content/uploads/2023/04/Transfusionists-Talk-2023-Mar.pdfDonna Berta RN BScN, Clinical Project Coordinator – Nursing, ORBCoN
Hemovigilance has been described as surveillance measures spanning the entire transfusion process from blood collection to blood recipient follow-up. Information on adverse transfusion effects is compiled to limit re-occurrence. The goal of reporting and evaluating adverse events is to improve transfusion safety.
Canada’s national hemovigilance system, Transfusion Transmitted Injuries Surveillance System (TTISS), was implemented by the Centre for Communicable Disease and Infection Control (CCDIC) of the Public Health Agency of Canada (PHAC) in 2001. Each province and territory report their transfusion reactions to TTISS.
In Ontario, the Ministry of Health commissions the McMaster Centre for Transfusion Research of McMaster University to coordinate the province’s TTISS actions (TTISS-ON). Participation is voluntary, but all Ontario hospitals have signed on to report moderate to severe transfusion reactions to blood components (red blood cells, platelets, plasma, cryoprecipitate) and plasma protein and related products (PPRP).
Blood component serious or unexpected transfusion reactions (posing a significant risk to patient safety) and those pertaining to the safety of the blood itself must be reported to Health Canada’s Canada Vigilance Program directly or via the manufacturer (Canadian Blood Services or Héma-Québec) in addition to TTISS-ON. Effective December 16, 2019 (as per Vanessa’s Law), PPRP serious adverse reactions must be reported directly to Health Canada’s Canada Vigilance Program as well as to the manufacturer of the product. Refer to the TTISS-ON website for their interactive Ontario Guide for Reporting Transfusion Reactions to identify the reporting requirements for each transfusion reaction.
A sentinel site model (where 29 Ontario hospitals have committed to report all transfusion reactions [minor (febrile non-hemolytic, delayed serologic, and allergic) reactions in addition to major, moderate to severe, transfusion reactions] is an integral component of the TTISS-ON program. These 29 Ontario hospitals represent approximately 35% of all blood components transfused in Ontario. This sentinel site transfusion reaction data along with Canadian Blood Services (CBS) disposition data are used to calculate the Ontario incidence or frequency of transfusion reactions by type and by blood component. Sentinel site data for the 2020 calendar year are displayed in Figure 1 and 2 and Table 1.
At annual hospital site visits, ORBCoN and CBS provide this data along with hospital specific data (based on transfusion reactions reported by that hospital) to highlight transfusion patient safety. Transfusion Medicine Services have reaction investigating and reporting accountabilities. Accordingly, transfusion prescribers and transfusionists are accountable to recognize and report transfusion reactions. Hospital specific data reports (graphs and tables) generated from TTISS-ON and CBS data should be reviewed by Transfusion and Quality committees and shared with front line care providers to support hemovigilance.
For more information about report generation resources, contact Joanne Duncan, TTISS-ON Coordinator (duncanj@mcmaster.ca).
Figure 1: 2020, Sentinel Sites, Minor (febrile non-hemolytic, delayed serologic, and allergic) Reactions
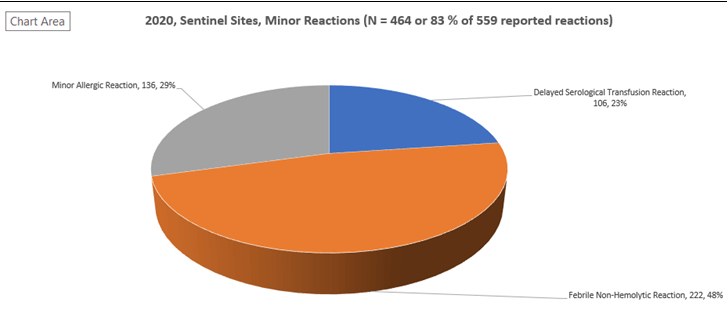
Figure 2: 2020, Sentinel Sites, Major (moderate to severe) Reactions
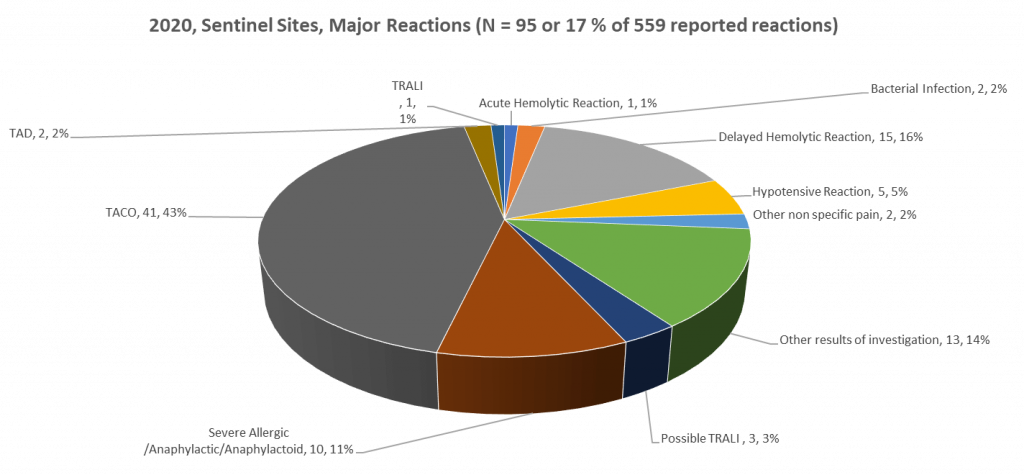
Table 1: 2020, Sentinel Sites: Transfusion Reaction Type per Blood Component and Incidence of Major, Minor, Any Reaction per Blood Component
| Number of Units Transfused | 113,696 | 21,657 | 16,705 | 152,058 |
|---|---|---|---|---|
| Red Blood Cells | Platelets | Plasma | Total | |
| Transfusion Reaction Type | ||||
| Major | ||||
| Acute Hemolytic | 1 | 1 | ||
| Bacterial Infection | 2 | 2 | ||
| Delayed Hemolytic | 15 | 15 | ||
| Hypotensive | 4 | 1 | 5 | |
| Other nonspecific pain | 2 | 2 | ||
| Other results of Investigation | 8 | 5 | 13 | |
| Possible TRALI | 1 | 1 | 1 | 3 |
| Severe Allergic/Anaphylactic/Anaphylactoid | 2 | 7 | 1 | 10 |
| TACO | 34 | 6 | 1 | 41 |
| TAD | 1 | 1 | 2 | |
| TRALI | 1 | 1 | ||
| Minor | ||||
| Delayed Serological | 105 | 1 | 106 | |
| Febrile Non-Hemolytic | 165 | 55 | 2 | 222 |
| Minor Allergic | 31 | 91 | 14 | 136 |
| Total | 369 | 170 | 20 | 559 |
| Incidence of Major Reaction | 1 in 1,672 | 1 in 941 | 1 in 4,176 | 1 in 1,600 |
| Incidence of Minor Reaction | 1 in 377 | 1 in 147 | 1 in 1,044 | 1 in 328 |
| Incidence of Any Reaction | 1 in 308 | 1 in 127 | 1 in 835 | 1 in 272 |
Acknowledgement:
Ontario Transfusion Transmitted Injuries Surveillance System (TTISS-ON) is gratefully acknowledged for the data provided.
References:
Canadian Blood Services (CBS) A guide to reporting adverse transfusion reactions [Internet]. [Ottawa], CBS; 2020 [cited 2022 Mar 7]. Available from:
https://professionaleducation.blood.ca/en/guide-reporting-adverse-transfusion-reactions
International Society of Blood Transfusion (ISBT). Haemovigilance [Internet]. [Amsterdam, the Netherlands], ISBT; 2022 [cited 2022 Mar 7]. Available from:
https://www.isbtweb.org/isbt-working-parties/haemovigilance.html
Ontario Transfusion Transmitted Injuries Surveillance System (TTISS-ON). Report an adverse reaction [Internet]. [Hamilton], TTISS-ON; 2022 [cited 2022 Mar 7]. Available from: https://ttiss.mcmaster.ca/?page_id=65
Celebrating National Medical Laboratory Week

Medical laboratories are at the centre of healthcare! Although you might not see them, #LabISEssential. On behalf of ORBCoN Happy National Medical Laboratory Week to all medical laboratory professionals for everything you do!
Register: CSTM 2022 Annual Conference

Register: Perinatal Consensus Conference

Featured Resource: IVIG Infusion Guide
Coming Soon: Transfusion Medicine Education Web Conference Archive Video
The 17th annual Transfusion Medicine Education Web Conference was held April 8th 2022. This event showcased dealing with different transfusion requirements for different types of patients with a massive bleed. If you missed the event it will be archived on the Transfusion Ontario website for viewing. Watch for it on transfusionontario.org in the coming week. We would like to extend our thanks to the organizers and the speakers for another great symposium. See you all next year!

Upcoming Event: University of Toronto Transfusion Medicine Rounds

April 28th, 2022 @ 12:00 pm – 1:00 pm
Transfusion Related Acute Lung Injury (TRALI), presented by Dr. Alexander Vlaar