September 2023

Before we begin
Newsletter: Did someone forward you this newsletter? Subscribe here
In This Issue
- Lab Wisely: Empowering Medical Laboratory Professionals
- ORBCoN 2022 Year Review
- Newsboard
- Upcoming Events
Lab Wisely: Empowering Medical Laboratory Professionals
Amanda VanSpronsen, MSc, BSc (MLS), MLT, PhD
In late 2018, the Canadian Society for Medical Laboratory Science (CSMLS) approached the
University of Alberta (UofA) to explore participation in the Choosing Wisely (CW) initiative. As
most medical laboratory professionals (MLPs) are aware, sometimes healthcare providers order
laboratory tests that aren’t necessary. This wastes time and resources, but can also lead to
patient harm in multiple ways, such as causing or exacerbating anemia. Despite this widespread
issue, little progress had been made until the inception of CW in 2012. CW collaborates with
various professional societies to develop recommendations for commonly mis-ordered medical
tests and procedures. For instance, “Don’t routinely measure Vitamin D in low-risk adults” from
Family Medicine, and “Don’t routinely perform preoperative testing for patients undergoing low
risk surgeries” from Internal Medicine have been published. Choosing Wisely Canada now
boasts partnerships with dozens of societies and lists hundreds of recommendations, and is
continually expanding. Healthcare professionals can access these recommendations and use
them to drive positive changes and improve test ordering appropriateness in their respective
domains.
Unsurprisingly, many of CW recommendations are about laboratory testing, but early on, there
was no medical laboratory society involvement. This prompted the CSMLS and UofA to unite
and spearhead a process to identify how MLPs contribute to the issue of excessive testing. By
the end of 2020, they finalized a list of seven recommendations that all MLPs should be aware
of:
- Don’t collect more blood than what is needed. Use short draw tubes, consider add-on
testing, and reduce or combine duplicate orders. - Don’t proceed with testing or reporting when sample quality or identification is suspect.
- Don’t collect extra blood tubes in anticipation of test orders.
- Don’t support repeat test ordering (re-testing) at a frequency that is not backed by
evidence. - Don’t routinely repeat critical results for most common analytes before reporting.
- Don’t support ordering system mechanisms that contribute to over-testing. Encourage
the development of an evidence-based utilization management program that may
include interventions such as unbundling order sets, reflex testing algorithms, and
decision-support technology. - Don’t allow standing orders for repeat testing without a stop or review date.
The medical laboratory science CWC list contains items that are within the influence and control
of MLPs, and are each backed by evidence
(https://choosingwiselycanada.org/recommendation/medical-laboratory-science/). In addition,
the CSMLS has created a website called Lab Wisely (https://labwisely.ca/), which contains
additional tools and resources, designed to help MLPs initiate change in their workplaces.
Notably, Lab Wisely features an impressive searchable database of all 400+ CWC recommendations that can be filtered by laboratory discipline and stage of testing. Currently, they are working on ‘Lab Wisely 2.0’ to develop even more MLP recommendations for CWC by the end of 2023. Stay tuned for updates!
Addressing the issue of inappropriately ordered laboratory tests is a longstanding challenge, and it will take considerable effort and time to move the dial. It starts with raising awareness among all healthcare professionals and patients, and then working together, both within and across disciplines, to make things better. CWC and the CSMLS are taking collaborative approaches, and there are many ways to get involved. For instance, CWC is currently leading the ‘Using Labs Wisely’ campaign, a national consortium of hospitals that are implementing interventions based on CWC recommendations, collecting and sharing data, and identifying key lessons that can be applied elsewhere. There are currently 104 hospitals participating with the next round of applications opening this year (https://choosingwiselycanada.org/hospitals/using-labs-wisely/). The CSMLS is inviting stories of test utilization improvement projects, and has a collection of case studies on the Lab Wisely website to showcase multiple ways that can MLPs contribute. (https://labwisely.ca/success-stories/) Share yours today! With the efforts leading to Lab Wisely, MLPs now have a powerful toolkit at their disposal. By empowering themselves with knowledge, advocating for change, and participating in initiatives like the ‘Using Labs Wisely’ campaign, professionals can make a tangible impact, improve testing practices, and reduce resource waste, which will benefit both patients and the healthcare system.
Newsboard
Registration Open
ORBCoN Fall Symposium
Bloody Easy 5.1
Available Now

ORBCoN 2022-23 Year Overview
Tracy Cameron, MLT, Regional Manager ORBCoN NE
The Ontario Regional Blood Coordinating Network (ORBCoN) is a program funded by the Ontario Ministry of Health (MOH) to support the provincial blood utilization strategy using the following five strategic goals:
- Utilization of blood components and products
- Educational Resources
- Inventory Management
- Communication
- Quality & Safety
ORBCoN, as a program, strives to continually improve the management and utilization of blood components and products in the province of Ontario to ensure that transfusion related patient care is safe, effective, and provincial resources are utilized appropriately and efficiently. ORBCoN’s mission and vision are executed through our five strategic goals while maintaining the network’s core values: accountability, leading practice, collaboration, quality, and patient safety.
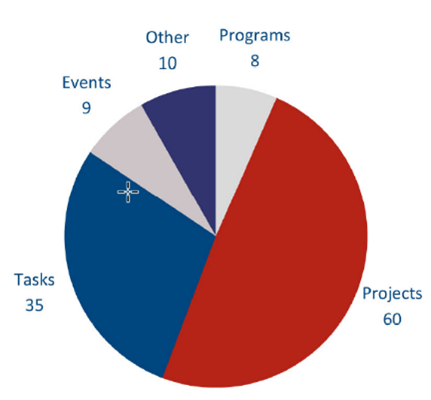
The 2022-23 work plan submitted to the MOH in September 2021, listed 113 high-level deliverables across the different ORBCoN goal categories. Deliverables have been categorized as projects, tasks, programs, events and other as outlined in figure 1.
ORBCoN has made significant progress in implementing the 2020 recommendations of the Office of the Auditor General of Ontario (OAGO) on Immune Globulin Utilization. One of these accomplishments is piloting an online MOH IG Request form to collect data on IG utilization-related variables.
ORBCoN is actively collaborating with data strategy partners through its participation in the provincial data strategy sub-committee to address access to data for measuring key indicators in Transfusion Medicine.
Despite the challenges posed by the pandemic, ORBCoN successfully organized six educational events, consisting of two live events and four online events. The tech assessment eLearning program was redeveloped and launched. Bloody Easy 5 handbook was published, and the patient pamphlet was reviewed, updated and published. The 1st “Transfusionist Talk” webinar series was broadcasted.

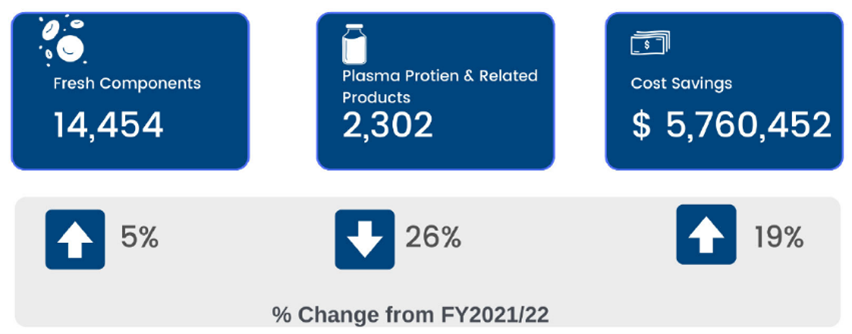
ORBCoN focused on contingency planning, monitoring outdate rates, and redistribution (figure 3) throughout the fiscal year of 2022-23. Red cell outdate rates continued to be very low through the pandemic even though the variability in demand fluctuated.
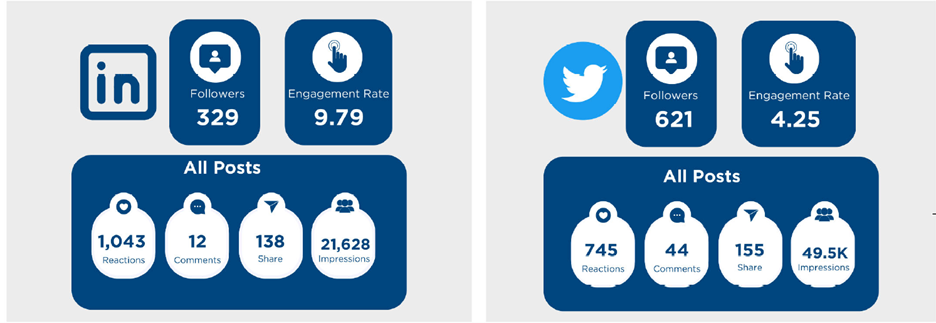
By leveraging the transfusion Ontario website, ORBCoN’s twitter and LinkedIn accounts, along with the annual site visits, ORBCoN continues to foster efficient and interconnected communication framework, ensuring transparency and timely information to stakeholders. Figure 4 shows how ORBCoN is using social media to provide timely information to stakeholders.
ORBCoN continued its commitment to achieving its quality and safety goals and is working collaboratively with other healthcare professionals to improve optimal frozen plasma utilization. The Bedside audit tool was rebuilt and piloted in preparation for the provincial audit. ORBCoN’s clinical coordinators began providing presentations

to healthcare providers in person again post pandemic at a few sites, and reviews of non conformances from Health Canada and from Accreditation Canada – Diagnostics reports continued.
ORBCoN would like to thank all our stakeholders and collaborators again this year for all the support in developing, implementing, and reviewing our resources that help ensure safe transfusions within Ontario.
Upcoming Events
Register
Transfusionists Talk: Transfusions Made Bloody Easy

Register
U of T TM Rounds

