A pathogen-reduced inventory at Canadian Blood Services with a focus on solvent detergent plasma
Kathryn Webert, MSc, MD, FRCPC
Medical Director and Special Advisor
Canadian Blood Services
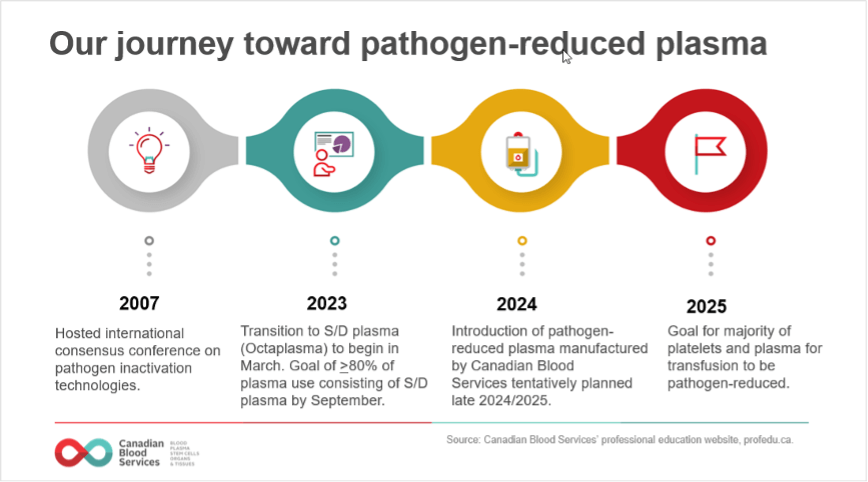
To provide an additional layer of product safety, Canadian Blood Services aims to introduce pathogen-reduced components and products, wherever possible (Figure 1). Our journey towards a pathogen-reduced inventory began in January 2022, when Canadian Blood Services began a staggered implementation of pathogen-reduced platelets.
In March 2023, the transition to pathogen-reduced plasma will begin with the widespread implementation of Octaplasma, a solvent detergent (S/D) treated plasma. In addition, implementation of another type of pathogen-reduced plasma, to be collected and manufactured by Canadian Blood Services using a different pathogen-inactivation technology and made available alongside S/D plasma, is tentatively planned for late 2024/25. Ultimately, it is expected that approximately 80% of plasma use will consist of S/D plasma and 20% will consist of Canadian Blood Services collected pathogen-reduced plasma. By 2025, the goal is for most platelets and plasma for transfusion to be pathogen-reduced.
Octaplasma is the commercial name for a pathogen-reduced form of frozen plasma manufactured by Octapharma. Octaplasma has been available only for special patient populations, but this will change as of March 2023, when it becomes available to all patients. Making it available to all patients is the most timely and cost-effective path towards a pathogen-reduced plasma supply.
When Octaplasma is manufactured, plasma from hundreds of donations is pooled. This step reduces antibodies that may be present in the plasma pool through dilution. The pooled plasma then undergoes sterile filtration steps resulting in the removal of leukocytes, as well as prions, parasites, and viruses. The plasma is treated with a combination of 1% tri(nbutyl)-phosphate, or TNBP, and 1% octoxynol. The detergent, octoxynol, interrupts the interactions between the molecules in viruses’ lipid coatings. Most enveloped viruses cannot exist without their lipid coating, so they are destroyed when exposed to these detergents. The solvent, TNBP, helps the reaction between the lipid coat and the detergent to happen more rapidly. The solvent and detergent reagents are removed by castor oil extraction (removes TNBP) and solid phase extraction (removes octoxynol). The plasma is then run through an affinity ligand resin column which specifically removes prion proteins. This step is considered effective for removing the infectious agent that causes variant Creutzfeldt-Jakob disease (vCJD) if present. The final product is packaged into blood bags with a standardized unit volume of 200 mL and frozen.
There are many benefits of S/D treatment: it reduces the residual risk of transfusion-transmitted infections that remain after donor screening and donation testing and reduces the risk of emerging or unknown pathogens that can be transmitted during transfusion to a patient. In addition, the literature suggests that S/D plasma may be associated with a decreased risk of adverse reactions, including allergic reactions and transfusion related acute lung injury (TRALI).1-5 The use of Octaplasma also benefits the blood system as it results in increased Canadian plasma available for fractionation.
On March 27, 2023, changes to the S/D plasma ordering process will be implemented, which will allow hospitals to order Octaplasma for all patients. By September 2023, all hospitals will be expected to order Octaplasma, with at least 80% of their plasma issues made up by Octaplasma by this date. This percent target will be guided by the experience of hospital customers as they transfuse more Octaplasma.
To assist hospitals during implementation steps, Canadian Blood Services is developing a series of educational materials which will be posted on our professional education website. Octapharma will also be making resources available.
Please contact Octapharma with any questions about Octaplasma and your local Hospital Liaison Specialist with any questions about Canadian Blood Services and plasma. Always refer to the Product Monograph for up-to-date information.6
References
- Saadah NH et al. Transition from fresh frozen plasma to solvent/detergent plasma in the Netherlands: comparing clinical use and transfusion reaction risks. Haematologica 105:1158-65 (2020).
- Liumbruno GM and Franchini M. Solvent/detergent plasma: pharmaceutical characteristics and clinical experience. J Thromb Thrombolysis 39:118-28 (2015).
- McGonigle AM et al. Solvent detergent treated pooled plasma and reduction of allergic transfusion reactions. Transfusion; 60:54-61 (2020).
- Klanderman RB et al. Reported transfusion-related acute lung injury associated with solvent/detergent plasma – A case series. Transfusion; 62: 594-99 (2022).
- Sachs UJ et al. White blood cell-reactive antibodies are undetectable in solvent/detergent plasma. Transfusion; 45:1628-1631 (2005).
- Octapharma. Octaplasma Product Monograph. October 21, 2022. Accessed at: https://www.octapharma.ca/en/therapies/product-overview
Newsboard
ORBCoN Tech Assessment

The ORBCoN Technologist Assessment program is now available on the new ORBCoN Learning Management System (LMS) and Surge Learning platforms.
A communication has been issued with instructions to provide site administrator information for access to the ORBCoN LMS and guidance for current Surge Learning clients.
If you have any questions or have not received the communication please reach out to alison.wendt@sunnybrook.ca
Provincial Bedside Audit of Blood Administration

Register: Transfusionists Talk: Transfusion Made Bloody Easy
Save the Date: 18th Annual TM Conference

Featured Resources
Frozen Plasma Toolkit
Upcoming Events
UofT TM Rounds

February 23, 2023 @12pm-1pm
ICTMG Behaviour change by Dr. Fabina Lorencatto & Dr. Justin Presseau