November 2020

Canadian Obstetrical Pediatric Transfusion Network (COPTN)
2018 Survey – Ontario Highlights
By Sheena Scheuermann MLT BTech, Regional Project Coordinator, ORBCoN
The Canadian Obstetrical Pediatric Transfusion Network (COPTN) is a sub-committee of the Canadian Society for Transfusion Medicine (CSTM). It was founded in 2017 and its mandate is to assess, analyze and strive to implement best practices in pediatric and obstetrical transfusion practice in Canada.
A national survey was sent out to the provinces with the objective to assess national practice related to perinatal testing and to provide feedback and guidance regarding best practice. The Canada-wide survey report was released in the spring of 2019. The provinces each received their individual results from the survey and the Ontario report is currently being worked on at ORBCoN.
One hundred and fifty-six (156) hospitals and laboratories were sent this survey in Ontario and all of the organizations responded, representing a 100% response rate in the province. Ontario’s responses represented 26.9% of the responses across Canada. Not all facilities within the province perform antenatal testing, 58.33% (91 /156 respondents) perform testing. Antenatal testing questions were asked of the 91 participants that stated testing was performed.
| Which of the following describe D testing performed for prenatal patients and/or females of child bearing potential (PP/FCBP) in your laboratory? | Ontario (N=91) | National (N=243) |
| Either a strong or weaker D in the routine test are reported and/or treated as positive without additional testing | 42.8% | 30.5% |
| A weaker than normal D in the routine test are reported and/or treated as negative without additional testing | 2.2% | 8.6% |
| Either a strong or weaker D in the routine D test, is repeated using a 2nd reagent and/or technique | 23.1% | 21.0% |
| A weaker than normal D in the routine D test, is repeated using a 2nd reagent and/or technique | 52.7% | 51.0% |
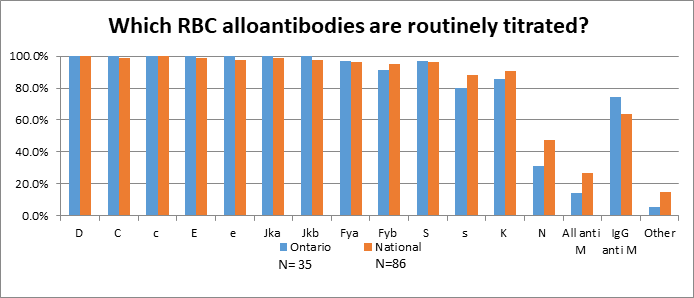
Sites who perform titration of clinically significant antibodies on site (n=35) were ask which antibodies were routinely titrated (figure.1). Out of the 35 sites performing titres on site the majority (88.6%) are performing them by tube saline indirect antiglobulin test (IAT). Thirteen (37.1%) reported they perform the titres at a frequency of monthly to 26 weeks then every two weeks thereafter or every two weeks if the titre increases two fold.
Sites were asked a question regarding cord blood samples. Testing performed on cord samples can be found in table 2.
| According to your Transfusion Medicine SOPs, what testing can be performed on cord blood samples | Ontario (n=95) | National (n=297) |
| ABO | 98.0% | 97.6% |
| RhD | 97.0% | 97.6% |
| Weak D testing | 91.0% | 88.2% |
| Direct antiglobulin (DAT) | 97.0% | 93.6% |
| Antibody screen | 19.0% | 17.2% |
| Maternal/passive anti-A/B (Reverse group IAT) | 20.0% | 20.2% |
| NaOH (or other substance) test to verify cord sample source | 15.0% | 7.1% |
| Crossmatch | 7.0% | 7.1% |
| Other | 8.0% | 9.8% |
Sites were asked if they perform testing for the detection and/or quantitation of fetal maternal hemorrhage (FMH), 48 sites in the province stated that they did. The initial detection for FMH was the Kleihauer-Betke (KB) test at 46.8% of the sites in the province (table 3.)
| What is your initial method for detection of FMH? | Ontario (n=47) | National (n=145) |
| Rosette | 42.60% | 48.3% |
| Kleihauer-Betke (KB) | 46.80% | 45.5% |
| Flow Cytometry | 2.10% | 2.1% |
| Other | 8.50% | 4.1% |
This survey consisted of 98 questions which could have been answered in many different pathways depending on what testing was performed at your site. We would like to thank the COPTN and CSTM and also all of the hospital sites that participated in this survey. We look forward to presenting a more detailed analysis of our provincial results in the new year. For more information on the COPTN please go to the CSTM website. https://www.transfusion.ca/Resources/Canadian-Obstetrical-and-Pediatric-Transfusion-Net
Administering RhIG: Vital Signs and Patient Monitoring
By Donna Berta RN BScN, Clinical Project Coordinator – Nursing, ORBCoN
Question:
Nurses are wondering if they are required to document vital signs (similar to when administering blood components, IVIG or albumin) for a 1500 IU dose of RhIG.
Are there special vital sign requirements for administering RhIG or do the same rules apply as when transfusing a blood component?
Answer:
The Canadian Standards Association, Standard CSA Z902 – Blood and Blood Components, which details the requirements to ensure safe transfusion practice, was revised effective March 2020. For blood component (red blood cells, platelets, plasma, and cryoprecipitate) transfusion, CSA Z902-20 sections 11.4.15-6 provide clear direction: “Recipient vital signs shall be recorded at least before transfusion, within 15 min after the start of transfusion and after transfusion. The recipient shall be observed during the transfusion and for an appropriate time thereafter for suspected adverse events”.1
For blood product (products derived from human blood or plasma and produced by a manufacturing process) transfusion, the information is not as explicit. CSA Z902-20 section 14.1.1-2 includes: “Clause 14 establishes requirements for … blood products and is intended to align the facility’s procedures for blood products with its procedures for blood components. … The procedures shall be designed to ensure that the blood product manufacturer’s instructions are followed”.1 An additional notation (CSA Z902-20 section 14.5) indicates “The requirement to address the same aspects recognizes that certain specific requirements applying to blood components … cannot be directly applied to blood products”.1
The current version (2017) of the Canadian Society for Transfusion Medicine published standards for hospital transfusion services does not list further guidance for vital signs or patient monitoring.2
The WinRho® SDF (RhIG brand available in Ontario) manufacturer’s product monograph does not stipulate vital sign assessment.3 However, the monograph advises “Following administration of WinRho® SDF for prophylaxis of Rh(D) immunization, patients should be kept under observation for at least 20 minutes for monitoring of potential adverse effects”.3 Potential adverse effects are rare and include: allergic or anaphylactic reactions (hives, rash, chest tightness, wheezing, shortness of breath, hypotension), discomfort or slight swelling at the injection site (if given intramuscularly) and minor increase in temperature.3 Note, this information from the WinRho® SDF monograph is specific to a dose of 1500 IU for prophylaxis of Rh(D) immunization (pregnancy, other obstetric conditions, transfusion of Rh(D) positive red blood cells or platelets to an Rh(D) negative patient). If WinRho® SDF is administered as treatment for Immune Thrombocytopenic Purpura (ITP), added patient monitoring details are described.3
Recommendations to translate this knowledge to clinical practice:
- Each Transfusion Medicine service should approve their facility’s procedure for vital signs and patient monitoring for blood product administration
- For RhIG administration for prophylaxis of Rh(D) immunization the procedure might include:
- Baseline vital signs prior to administration
- Vital signs 15 minutes following administration
- Observation for potential adverse effects for 20 minutes following administration
References
- National Standard of Canada Canadian Standards Association (CA) Blood and blood components. Toronto ON; 2020 Mar 24; cited 2020 Oct 9. 162 p. Report No.: CAN/CSA-902:20. Available from: https://community.csagroup.org/docs/DOC-126295
- Canadian Society for Transfusion Medicine (CA) Standards for hospital transfusion services. Markham ON; 2017 Apr 1; cited 2020 Oct 9. 102 p. Report No.: Version 4. Available from: http://www.transfusion.ca/Resources/Standards
- Saol Therapeutics Research Limited Distributor (in Canada) Emergent BioSolutions Canada Inc. WINRHO® SDF product monograph [Internet]. [Place unknown], [Publisher unknown]; 2020 Mar 31 [cited 2020 Oct 9 ]. Available from: https://winrho.com/pdfs/WinRho%20PM-EN042420.pdf