Are we closer to seeing the end of cryoprecipitate in Ontario?
Jeannie Callum, BA, MD, FRCPC
Director of Transfusion Medicine, Kingston Health Sciences Centre
In October of 2019, the results of the FIBRES trial were simultaneously released in both JAMA and at the plenary session of the AABB annual meeting.1 The conduct of the FIBRES trial was a major achievement for hospital blood bank technologists and cardiac surgical teams at 11 hospitals across Canada. The trial determined that fibrinogen concentrate was non-inferior to cryoprecipitate for hemostatic control of cardiac surgery-related bleeding and had a similar safety profile. In subgroup analysis, patients undergoing elective surgery were found to have superior hemorrhage control with fibrinogen concentrate, compared to cryoprecipitate. This was a major advance towards the transition to pathogen reduced blood products, which had been supported at the pathogen reduction consensus conference in Toronto in 2007.2 Indeed, Canadian Blood Services took Canada one step further in this important safety journey in 2022 with the introduction of pathogen-reduced platelet concentrates.
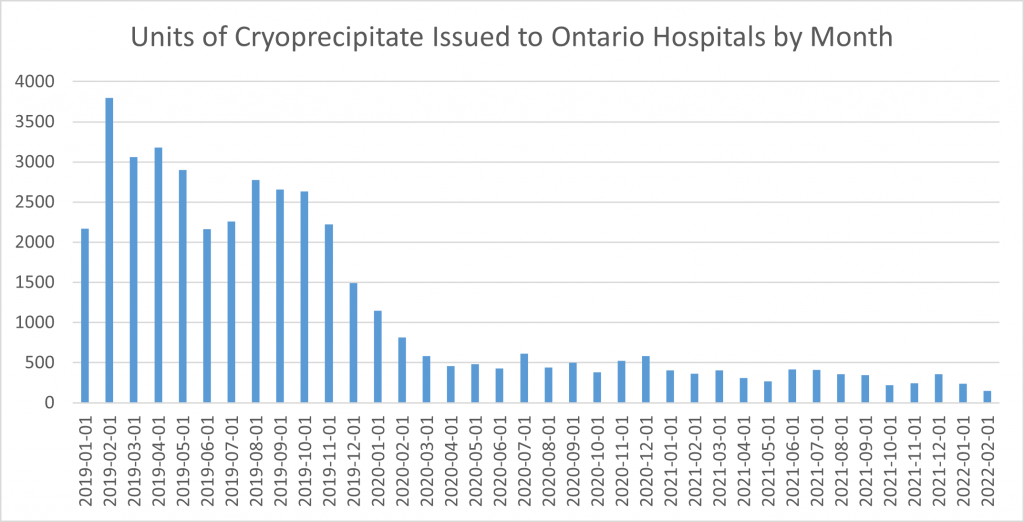
So where are we almost 30 months later after the publication of the FIBRES trial in the transition to a safer fibrinogen replacement product? Ontario turned on a dime after the FIBRES trial was published, with the number of cryoprecipitate units issued 8 weeks later in January 2020 already in half. In February of 2022, only 148 units or approximately 15 adult doses were issued to the remaining 12 hospitals in Ontario still ordering cryoprecipitate as a fibrinogen source. The majority of these 12 hospitals are non-academic hospitals, suggesting that process change rather than some inherent belief in the superiority of cryoprecipitate is resulting in a delay in transitioning. Change during COVID-19 is not easy. In addition, almost every transfusion laboratory is struggling with staffing due to shortages in medical laboratory technologists. A few of these 12 hospitals also have pediatric patients and are ordering single units suggesting reluctance to extrapolate the data from adult trials to pediatric patients. Fortunately, there have been two randomized trials comparing cryoprecipitate to fibrinogen concentrate in pediatric cardiac surgical patients with similar outcomes for efficacy and safety.3,4 One would hope we would prioritize pediatric patients to getting access to a safer fibrinogen replacement, since these patients are most likely to live long enough to be affected by a novel blood borne pathogen. Dr. Steven Kleinman estimated the economic impacts of a novel blood borne pathogen and the estimated number of affected patients in Canada; the estimates are staggering.5
In addition, to the paramount safety concern with the use of a non-pathogen reduced product when a safer product is available, cryoprecipitate has other negative characteristics we need to consider. First, Canadian Blood Services must collect B2-pack whole blood for their manufacture. Nancy Heddle has raised the concern in an article in Lancet Haematology in 2016 that the outcome for the recipient of these matching B2 red cells may be inferior.6 Second, when we utilize plasma for the manufacture of cryoprecipitate, the residual plasma cannot be used for either transfusion or for the manufacture of derivatives. Third, the B2 pack production line cannot be used to produce platelet concentrates. Fourth, cryoprecipitate is frozen and therefore cannot be easily redistributed if not used before impending expiration. Fifth, cryoprecipitate must be thawed and pooled before issue (along with a lot of computer clicks) delaying issue to hemorrhaging patients. This is also workload our dwindling supply of technologists must bear. Lastly, we have no idea what the patient safety risk of the impurities in cryoprecipitate may be for our patients (platelet microparticles, factor VIII, von Willebrand factor, etc.). Indeed, concern for an increased risk of thromboembolic complications has been raised by two papers.7,8
Dr. Judith Pool invented “Pool’s cryoprecipitate” in 1964 as a treatment for patients with congenital factor VIII deficiency.9 It later found a common use for acquired hypofibrinogenemia.10 Its use for the last 60 years for fibrinogen replacement has undoubtedly saved many lives, although unfortunately it has also been implicated in transmissible infections, including one high profile case (Blood money | Maclean’s | MARCH 28, 1994 (macleans.ca)). Most European countries have completed the transition to fibrinogen concentrates11 and it is reassuring to see that Ontario is almost there.
Figure 1. Units of cryoprecipitate issued to Ontario hospitals by month over the last 3 years.
References:
- Callum J, Farkouh ME, Scales DC, Heddle NM, Crowther M, Rao V, Hucke HP, Carroll J, Grewal D, Brar S, Bussieres J, Grocott H, Harle C, Pavenski K, Rochon A, Saha T, Shepherd L, Syed S, Tran D, Wong D, Zeller M, Karkouti K, Group FR. Effect of Fibrinogen Concentrate vs Cryoprecipitate on Blood Component Transfusion After Cardiac Surgery: The FIBRES Randomized Clinical Trial. JAMA 2019;322: 1966-76.
- Webert KE, Cserti CM, Hannon J, Lin Y, Pavenski K, Pendergrast JM, Blajchman MA. Proceedings of a Consensus Conference: pathogen inactivation-making decisions about new technologies. Transfus Med Rev 2008;22: 1-34.
- Galas FR, de Almeida JP, Fukushima JT, Vincent JL, Osawa EA, Zeferino S, Camara L, Guimaraes VA, Jatene MB, Hajjar LA. Hemostatic effects of fibrinogen concentrate compared with cryoprecipitate in children after cardiac surgery: a randomized pilot trial. J Thorac Cardiovasc Surg 2014;148: 1647-55.
- Downey LA, Andrews J, Hedlin H, Kamra K, McKenzie ED, Hanley FL, Williams GD, Guzzetta NA. Fibrinogen Concentrate as an Alternative to Cryoprecipitate in a Postcardiopulmonary Transfusion Algorithm in Infants Undergoing Cardiac Surgery: A Prospective Randomized Controlled Trial. Anesth Analg 2020;130: 740-51.
- Kleinman S, Cameron C, Custer B, Busch M, Katz L, Kralj B, Matheson I, Murphy K, Preiksaitis J, Devine D. Modeling the risk of an emerging pathogen entering the Canadian blood supply. Transfusion 2010;50: 2592-606.
- Heddle NM, Arnold DM, Acker JP, Liu Y, Barty RL, Eikelboom JW, Webert KE, Hsia CC, O’Brien SF, Cook RJ. Red blood cell processing methods and in-hospital mortality: a transfusion registry cohort study. Lancet Haematol 2016;3: e246-54.
- Myers SP, Brown JB, Leeper CM, Kutcher ME, Chen X, Wade CE, Holcomb JB, Schreiber MA, Cardenas JC, Rosengart MR, Neal MD, group Ps. Early versus late venous thromboembolism: A secondary analysis of data from the PROPPR trial. Surgery 2019;166: 416-22.
- Roy A, Stanford S, Nunn S, Alves S, Sargant N, Rangarajan S, Smith EA, Bell J, Dayal S, Cecil T, Tzivanakis A, Kruzhkova I, Solomon C, Knaub S, Moran B, Mohamed F. Efficacy of fibrinogen concentrate in major abdominal surgery – A prospective, randomized, controlled study in cytoreductive surgery for pseudomyxoma peritonei. J Thromb Haemost 2020;18: 352-63.
- Swenson E, Hollenhorst MA. Dr Judith Graham Pool and the development of cryoprecipitate. Transfusion 2021;61: 1676-7.
- Callum JL, Karkouti K, Lin Y. Cryoprecipitate: the current state of knowledge. Transfus Med Rev 2009;23: 177-88.
- Nascimento B, Goodnough LT, Levy JH. Cryoprecipitate therapy. Br J Anaesth 2014;113: 922-34.
Celebrating National Nursing Week

ORBCoN celebrates National Nursing Week, many thanks to every nurse for your compassion and dedication
See you at CSTM 2022
Hope to see you at the 2022 CSTM Conference and be sure to visit ORBCoN at our booth #114 !
Friendly members of our team will be available at the booth to network and answer any transfusion medicine questions you may have.

Register: CSTM 2022 Annual Conference

Featured Resource: Bug-free Platelets video
