Spotlight on Ferric Derisomaltose
Alanna Howell, RN
ONTraC Program Manager
The Global Health Burden 2019 Study estimated that there were 1.8 billion cases of anemia in the world and anemia accounted for 50.3 million years lived with disablility.1 Iron deficiency is known to be the most common type of anemia.
For several decades intravenous (IV) iron has been used as a successful treatment modality for iron deficiency and iron deficiency anemia (IDA). It is an option when oral iron is not effective or poorly tolerated or when there is a need to correct anemia rapidly. IV iron is used in various hospital specialty groups including cardiac, oncology, gastroenterology, gynecology and obstetrics. The use of IV iron has expanded in recent years and is now a part of perioperative patient blood management programs.
Earlier IV iron formulations were associated with serious reactions caused by a rapid iron release. In recent years newer formulations have come on the markets which are deemed to be safer. They contain carbohydrate cores which bind elemental iron more tightly resulting in a slower, controlled release and fewer reactions.2 One of these newer formulations is ferric derisomaltose (Monoferric®) which was formerly known as iron isomaltoside 1000. It is a third – generation IV iron compound. It allows for high dose infusion of up to 20 mg/kg or maximum of 1,500 mg administered as an infusion over 30 minutes or more in one visit versus multiple visits. First introduced in Europe in 2009, it was approved by Health Canada in 2018. The table below lists the indications and methods of administration for ferric derisomaltose in Canada.3
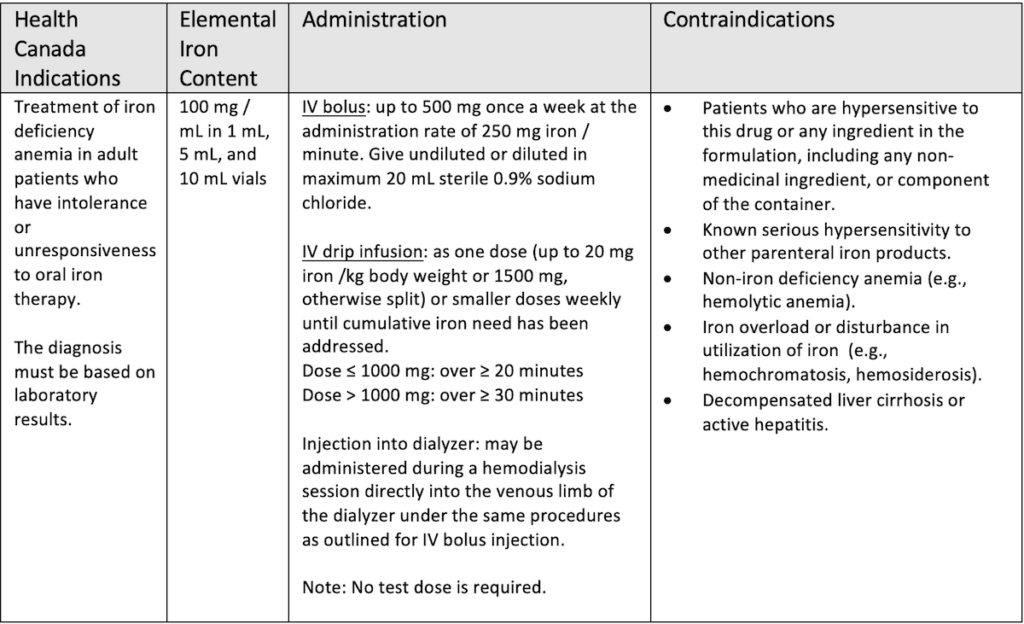
Ferric derisomaltose has shown good efficacy and safety in clinical trials. PROPOSE (N=351), FERWON-Nephro (N=1538), PROVIDE (N=511) and FERWON – IDA (N=1512) compared iron isomaltoside 1000 to iron sucrose in patients with IDA. All of these trials showed iron isomaltoside 1000 to be non-inferior to iron sucrose in raising and/or maintaining hemoglobin levels.,4,5,6
The recommended total dose is typically between 1000 mg and 1500 mg. The dose may be calculated using the Ganzoni formula or by using a simplified table that can be found within the product monograph. The cost of ferric derisomaltose is $45 per mL leading to a drug cost of $450 for a 1000 mg course of therapy, comparable to a three dose course of Venofer, an iron sucrose. The list below shows various ways the cost of the drug may be covered for patients in the province of Ontario.
- Self pay
- Private Insurance – DIN #02477777
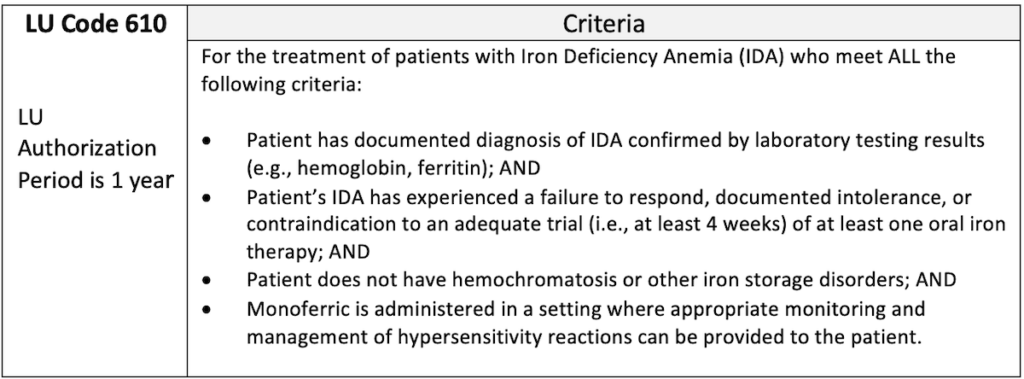
- Limited Use (LU) benefit funded through the Ontario Drug Benefit program The LU criteria is shown in the table below7:
- Canada Veterans Affairs formulary coverage as a standard benefit
- Government of Canada Non-Insured Health Benefit (NIHB) program as an open benefit
The use of ferric derisomaltose is becoming more popular. It provides a complete or near-complete replacement in a single infusion and is generally well tolerated. This results in improved convenience for the patient, shorter chair times, reduced number of infusions needed and a faster correction of anemia.
References
- Safiri S, Kolahi AA, Noori M, Nejadghaderi SA, Karamzad N, Bragazzi NL, Sullman MJM, Abdollahi M, Collins GS, Kaufman JS, Grieger JA. Burden of anemia and its underlying causes in 204 countries and territories, 1990-2019: results from the Global Burden of Disease Study 2019. J Hematol Oncol. 2021 Nov 4;14(1):185. doi: 10.1186/s13045-021-01202-2.
- Auerbach M, Macdougall IC. Safety of intravenous iron formulations: facts and folklore. Blood Transfus. 2014 Jul;12(3):296-300. doi: 10.2450/2014.0094-14..
- Monoferric (ferric derisomaltose) Canadian Product Monograph Pharmacosmos A/S Nov 2022.
- Derwman, R. et al. A randomized trial of iron isomaltoside versus iron sucrose in patients with iron anemia. AmJHematol.92, 286-291 (2017)
- Auerbach M. et al. A prospective, multi-center, randomized comparison of iron isomaltoside 1000 versus iron sucrose in patients with iron deficiency anemia; the FERWON-IDA trial. Am J. Hematol. 94, 1007 -1014 (2019)
- CADTH Clinical Review Report: Iron Isomaltoside 1000 ( Monoferric): Pharmacosmos A/S): Indication: For the treatment of iron deficiency anemia in adult patients who have intolerance or unresponsiveness to oral iron therapy (May 2020)
- Ontario Drug Benefit Formulary /Comparative Drug Benefit. Available online at https://www.formulary.health.gov.on.ca/formulary/
Did you know?

Check out our new and improved IVIG/BMI app!
Upcoming Events
CBS/ORBCoN Technical Dry Workshop

18th Annual TM Webconference

Transfusionists Talk: Transfusion Made Bloody Easy
UofT TM Rounds

March 23, 2023 @12pm-1pm
Virtual – Whole Blood Debate by Dr. Nick Crombie, Dr. Mark Yazer and Dr. Jeannie Callum – moderator