January 2021

Obstetric MHP
Dr. Robert Jee MD FRCPC
Assistant Professor, University of Ottawa
Department of Anesthesiology and Pain Medicine,
The Ottawa hospital
Obstetric hemorrhage is a leading cause of maternal mortality, the majority of which is attributable to postpartum hemorrhage (PPH). In many cases these deaths are considered preventable. Physiologic changes in pregnancy make this population unique in terms of hemorrhage and transfusion management. The gravid uterus is a remarkably vascular organ with blood flow nearing a liter per minute at term; massive hemorrhage and coagulopathy can develop rapidly. Timely resuscitation facilitated by a massive hemorrhage protocol (MHP) is essential.
There are several important considerations when tailoring a MHP to the obstetric population. It is worth noting that parturients deliver in highly variable settings across Ontario. In many centres, birthing units are in more remote hospital locations, away from the main operating rooms, intensive units, and emergency departments where massive hemorrhage and transfusion more commonly occur. Larger centers may see massive hemorrhage regularly and be better rehearsed in its management, whereas most centres with lower delivery rates will see this type of event infrequently. It is therefore key to have a rehearsed, protocol driven, standardized MHP with a streamlined approach for activating it to ensure prompt delivery of blood products.
A standardized hospital MHP requires some tailoring for the obstetric population. Pregnancy is a time of unique physiology, and this includes a shift to a hypercoagulable state. There is an increase in most coagulation factor levels, most notably fibrinogen levels increase two-fold by term. What can appear as a normal fibrinogen level outside of pregnancy may be one that is markedly low for a parturient. A low fibrinogen level in the context of hemorrhage can be a marker for impending coagulopathy and massive hemorrhage. An obstetric MHP should include guidance for early testing of fibrinogen levels and maintaining levels > 2 gm/L. Earlier protocolized replacement of fibrinogen should be considered in the obstetric population. It is worth noting that coagulopathy is usually not the initial cause of maternal hemorrhage, and the development of a coagulopathy is often multifactorial. Aetiologies that may contribute to maternal coagulopathy may include things like placental abruption, infection, or rapid volume resuscitation while awaiting blood products.
Massive hemorrhage in parturients is often unanticipated and can develop precipitously. Healthcare providers are notoriously poor at estimating blood loss at delivery, with a tendency to underestimate actual blood loss. Initial resuscitation often precludes the use of laboratory guided transfusion, and blood products are typically delivered in predetermined ratios with a RBC:Plasma ratio of 2:1. Point of care testing devices that measure hemoglobin and INR can help guide initial transfusion. Most obstetric centres in Ontario do not have TEG or ROTEM devices at their disposal to guide transfusion management.
It is worth recognizing that an obstetric MHP functions in conjunction with PPH protocols. Both have common goals of attaining source control of bleeding, providing appropriate resuscitation, and avoiding further development of coagulopathy. Birthing units have existing policies in place that address both medical and surgical management of PPH. These would include treatment options like the use of uterotonic agents, anti-fibrinolytics, active patient warming, and surgical escalation options including uterine tamponade balloons, ligation of the uterine blood supply, and hysterectomy if required. An obstetric MHP needs to be considered in the greater context of obstetric patient care. It is ultimately a temporizing measure until source control can be achieved and laboratory guided transfusion can occur.
When developing and implementing a MHP for your hospital, it is important that consideration be given to special populations, including the obstetric population. Adapting the protocol to the unique considerations of this population will help ensure success of the protocol and ideally improve patient outcomes.
Save the dates:
April 14th: 16th Annual TM Education Web Conference

April 30th: Ontario’s First Recommendations for Massive Hemorrhage Protocol Virtual Sumposium

An update on the ORBCoN Standardized Pediatric Massive Hemorrhage Protocol
By Kimmo Murto MD1,2, Suzanne Beno MD3,4, Mark McVey MD4,5, Wendy Owens ART, B Comm6 and Lani Lieberman MD7,8
1Associate Professor, University of Ottawa, Department of Anesthesiology & Pain Medicine; 2Children’s Hospital of Eastern Ontario Research Institute; 3Associate Professor, University of Toronto, Department of Paediatrics, Division of Emergency Medicine; 4Hospital for Sick Children; 5Assistant Professor, University of Toronto, Department of Anesthesiology & Pain Medicine; 6Program Manager, Ontario Regional Blood Coordinating Network; 7Assistant Professor, University of Toronto, Department of Laboratory Medicine & Pathology; 8University Health Network: Toronto General Hospital
In children, massive hemorrhage is a leading cause of preventable death in trauma and in the operating room, massive hemorrhage is the leading cause for cardiac arrest.1-4 Six per 10,000 live births result in an emergency blood product request for a neonate experiencing massive hemorrhage in utero or during birth from placental abruption/placenta previa with a mortality rate of 35%.5 Similar to adults, the in-hospital event rates of simple red blood cell (RBC) transfusions or larger volume RBC transfusions are, approximately 15 and 3%, respectively.6,8 The care provided to children experiencing massive hemorrhage is highly variable in part due to limited access to evidence-based massive hemorrhage protocols (MHPs), particularly in community settings.9 Care provided to children experiencing massive hemorrhage needs to be improved. In response, an ORBCoN sponsored Ontario-wide roll-out of a standardized massive hemorrhage protocol (MHP) and associated tool-kit for adults and children is planned for 2021. Pediatric MHPs have demonstrated improved system resource utilization such as, speed of blood product delivery to the bedside and decreased exposure to blood products in trauma settings. However, in contrast to adults, evidence for outcome benefits for children in both pediatric trauma and surgical settings remain elusive.10-14
The aim of this article, the first of a two-part series, is to briefly highlight key updates contained within the soon to be released ORBCoN-sponsored standardized pediatric MHP. The second article will address the “value proposition” to implement a pediatric MHP, particularly in a community setting. The reader is encouraged to read the associated MHP tool-kit document for management of children (≤ 12 years and < 40 kg) to gain an in-depth understanding of the background and references associated with these and other key recommendations.
Key pediatric MHP updates
A standardized province-wide pediatric MHP is the first step towards removing unwanted variation in care towards improving patient outcomes in all Ontario hospitals. Pediatric MHP best practices were determined by an ORBCoN-sponsored interdisciplinary working group (WG) and MHP Steering Committee from both Ontario community and tertiary care settings. Treatment guidance is based on pediatric observational data or extrapolated from the adult literature as prospective data availability is limited.15 Key recommendations by the pediatric WG are as follows:
Re-define massive hemorrhage triggers for activation in children: The majority of MHP are activated at physician discretion based on various factors. The most-widely accepted evidence-based definition of massive hemorrhage (40 ml/kg of blood products administered over 24 hours),16 has been replaced with an anticipated 40 ml/kg estimated/actual administration of red blood cells (RBCs) within three hours, as this time period is when most deaths occur.17,18
Administer blood products and crystalloids on a per kg basis to avoid fluid overload: Blood products should be administered in children on a ml/kg basis (RBCs, frozen plasma and platelets-20, 10-20 and 10 ml/kg, respectively) to avoid inadvertent volume overload.
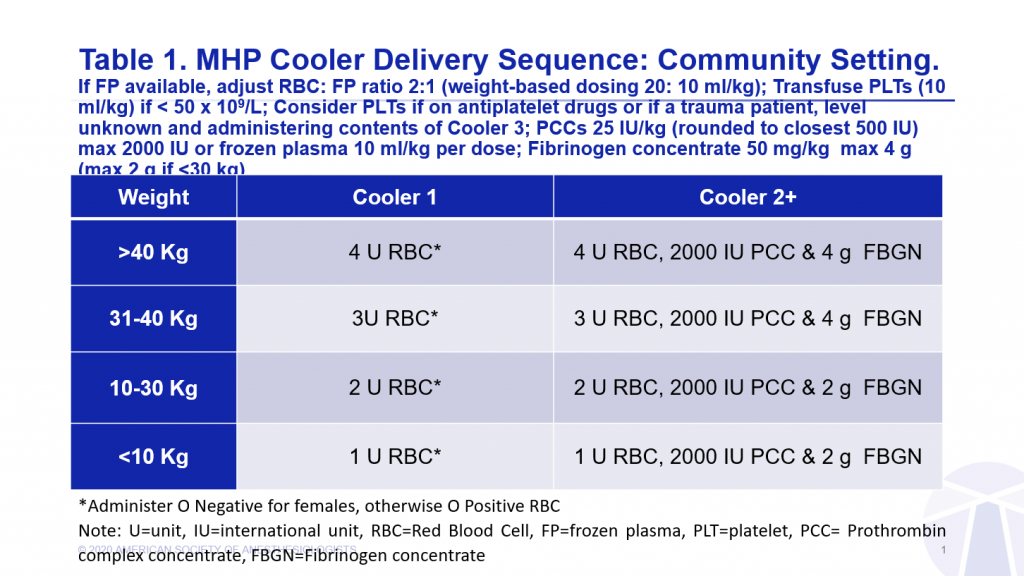
Avoid premature administration of frozen plasma: It is recommended that the first box of blood product administered to children, as in adults, should contain only RBCs during initial formula (ratio) driven resuscitation.
Remember Platelet administration should be goal-based and not formula driven: Prophylactic administration of platelets to reduce bleeding risk is controversial in children.33-35,43,44
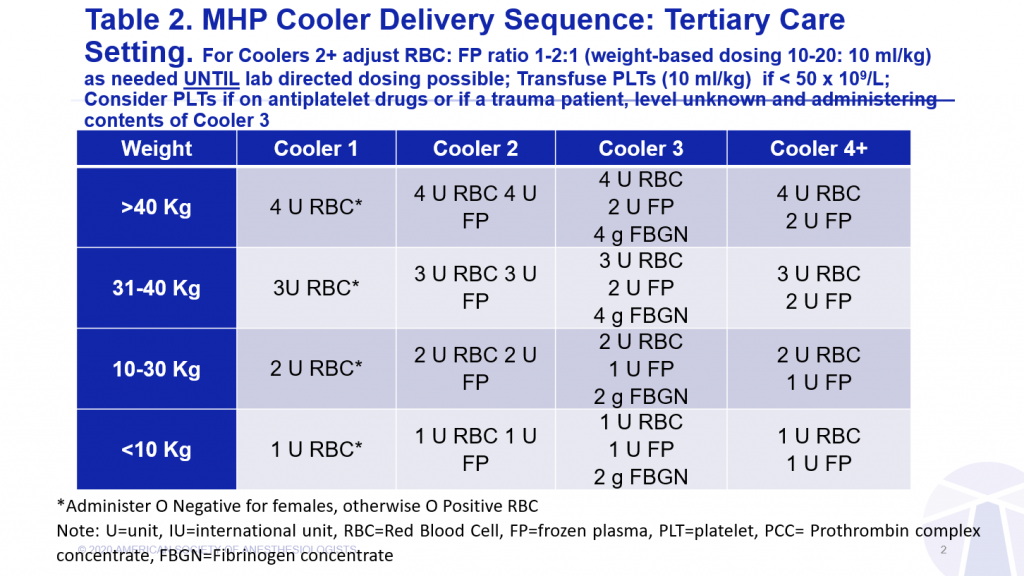
Adopt similar resuscitation targets in children and adults: Pediatric resuscitation target thresholds for hemoglobin (≥ 80 g/L), INR (< 1.8), fibrinogen (≥ 1.5 g/L) and platelets (≥ 50 x109/L) should be similar to adults in most children under 12 years old, given results of pediatric liberal versus restricted RBC transfusion trials and changes in the coagulation system being most marked in the first six-months of life.50-56
Avoid hypothermia: Considering the morbidity (i.e. increased risk of coagulopathy, arrhythmias, acidosis and transfusion) and mortality associated with hypothermia in massively bleeding children, they should receive interventions aimed to prevent hypothermia (goal >36°C).60-63
Monitor potassium, blood glucose and calcium in children: Hyperkalemia due to RBC transfusion is second only to hypovolemia from blood loss as the most common causes of cardiac arrest in the pediatric perioperative environment.4
Remember Tranexamic acid (TXA) administration in the pediatric trauma patient is not a standard of practice: Although prospective evidence of benefit or harm is not yet available for the use of anti-fibrinolytics such as TXA in children (< 16 years old) with trauma, there may be benefit (e.g. decreased blood loss) with little risk of harm (e.g. seizures) when administered within 3 hours of injury.
Transport and handover to a definitive care setting should be initiated early: Infants and children (< 13 years old) subject to or are at risk of MHP activation should receive specialized care.75,76 Children cared for in a community hospital setting should trigger early/urgent consultation with a specialized service using a “Situation, Background, Assessment and Recommendation” (SBAR) hand-over tool.
Conclusion
Given the inherent variability in pediatric MHPs, there is an opportunity to optimize patient outcomes by standardizing their care. This is ideal in an often chaotic and high-stakes environment found in both low trauma-volume community and busy tertiary care hospital settings. The protocol must account for a child’s immature body habitus and smaller size, mechanistic differences in trauma, unique indications for a higher RBC transfusion trigger and a predisposition to hypothermia and hyperkalemia compared with adults. Surrogate adult evidence suggests that in addition to improved patient outcomes, a reduction in complications and a potential for cost savings are to be expected,77 however, in the pediatric setting there remain unresolved issues. More robust pediatric specific evidence is required to determine evidence-based triggers for MHP activation and appropriate blood product ratios and timing of plasma and platelets especially in the setting of TBI. Further, while TXA is beneficial in the elective perioperative setting, advantage in the pediatric trauma setting is still to be determined. Finally, investigation of appropriate fluid, vasoactive agent and pain management strategies and means to modulate the associated systemic inflammatory response towards improved patient outcomes are still needed.
Upcoming Event:
January 28th: “SCD: Genotyping/alloimmunization prevention and management” presented by Dr. Stella Chou.
